News und Analysen

Grifols Procleix ArboPlex Assay® erhält die CE-Zertifizierung – der erste und einzige 4-in-1-Nukleinsäuretest zum Nachweis von Arboviren
- Der In-vitro-Nukleinsäuretest von Grifols weist vier Arten von Arboviren nach und trägt dazu bei, das Risiko transfusionsbedingter Infektionen zu verringern
- Arboviren stellen eine wachsende

Cresemba®-Verkaufszahlen in Lateinamerika lösen erste Umsatzmeilensteinzahlung an Basilea aus
Allschwil, 19. Januar 2024
Basilea Pharmaceutica AG, Allschwil (SIX: BSLN), ein biopharmazeutisches Unternehmen mit bereits vermarkteten Produkten und dem Ziel, Patienten zu helfen, die an
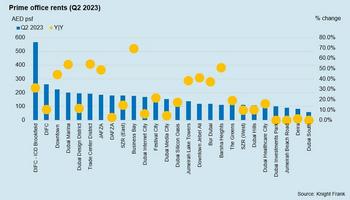
Dubai Office Demand Rises 23% To 580,000 Sqft In H1 2023
Dubai | 3rd August 2023 – Knight Frank, the leading global real estate consultancy, unveils its Dubai Office Market Review – Summer 2023 highlighting the robust demand for commercial office space

DGAP-News: RedHill Biopharma veröffentlicht Highlights aus Q1/2022: Im Plan zu einem positiven operativen Cashflow in H2/22

Lysogene Secures a €4.3 Million Non-dilutive Financing From Bpifrance to Support Its Development
Regulatory News:
Lysogene (FR0013233475 – LYS) (Paris:LYS), a phase 3 gene therapy platform Company targeting central nervous system (CNS) diseases, announced today that it has secured a €4.3

GenSight Biologics to Host Key Opinion Leader Webcast on Outcomes Among Compassionate Use Patients Treated Bilaterally with LUMEVOQ® in the US
Regulatory News:
GenSight Biologics (Euronext: SIGHT, ISIN: FR0013183985, PEA-PME eligible), a biopharma company focused on developing and commercializing innovative gene therapies for retinal

GenSight Biologics Reports Second Patient Case Showing Significant Visual Recovery after GS030 Optogenetic Treatment
GenSight Biologics (Euronext: SIGHT, ISIN: FR0013183985, PEA-PME eligible), a biopharma company focused on developing and commercializing innovative gene therapies for retinal neurodegenerative

GenSight Biologics to Present New Clinical Data of LUMEVOQ® and GS030 Gene Therapies at the American Academy of Ophthalmology 2021 Meeting
Regulatory News:
GenSight Biologics (Euronext: SIGHT, ISIN: FR0013183985, PEA-PME eligible), a biopharma company focused on developing and commercializing innovative gene therapies for retinal

Lysogene Announces a Presentation of its LYS-GM101 Program at the ESGCT 2021
Regulatory News:
Lysogene (FR0013233475 – LYS) (Paris:LYS), a phase 3 gene therapy platform Company targeting central nervous system (CNS) diseases, announces today that it will give an oral

GenSight Biologics Announces FDA Grant of Fast Track Designation for Optogenetic Therapy GS030 as Treatment for Retinitis Pigmentosa
Regulatory News:
GenSight Biologics (Euronext: SIGHT, ISIN: FR0013183985, PEA-PME eligible), a biopharma company focused on developing and commercializing innovative gene therapies for retinal

ABIONYX Pharma Initiates Discussions With IRIS Pharma, a World Leader in Preclinical and Clinical Ophthalmology Research, With a View to a Possible Strategic Deal
Regulatory News:
ABIONYX Pharma (Paris:ABNX) (FR0012616852 - ABNX - PEA PME eligible), a next-generation biotech company dedicated to the discovery and development of innovative therapies

GenSight Biologics Announces the Publication of a Review of Gene Therapy Trials for LHON in International Ophthalmology Clinics
Regulatory News:
GenSight Biologics (Euronext: SIGHT, ISIN: FR0013183985, PEA-PME eligible), a biopharma company focused on developing and commercializing innovative gene therapies for retinal

GenSight Biologics Announces Positive Data Safety Monitoring Board Review of PIONEER Phase I/II Clinical Trial of GS030 as Optogenetic Treatment for Retinitis Pigmentosa
Regulatory News:
GenSight Biologics (Euronext: SIGHT, ISIN: FR0013183985, PEA-PME eligible), a biopharma company focused on developing and commercializing innovative gene therapies for retinal

GenSight Biologics Announces Publication Analyzing Visual Parameters of ND4-LHON Subjects Before LUMEVOQ® Treatment in Phase III Trials
Regulatory News:
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20210908006154/en/
Figure 1. BCVA in untreated ND4-LHON patients as a

UK MHRA Grants GenSight Biologics’ LUMEVOQ® Ophthalmic Gene Therapy Promising Innovative Medicine Designation
GenSight Biologics (Paris:SIGHT) (Euronext: SIGHT, ISIN: FR0013183985, PEA-PME eligible), a biopharma company focused on developing and commercializing innovative gene therapies for retinal

GenSight Biologics Announces Publication of RESTORE Study Data Demonstrating Sustained Efficacy 3 Years After Unilateral Injection of LUMEVOQ®
Regulatory News:
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20210830005575/en/
Figure 1. Evolution of best-corrected visual acuity

Lysogene Announces First Patient in the United States Dosed with LYS-GM101 Investigational Gene Therapy for the Treatment of GM1 Gangliosidosis
Regulatory News:
Lysogene (FR0013233475 – LYS) (Paris:LYS), a phase 3 gene therapy platform company targeting central nervous system (CNS) diseases, today announced dosing of the first patient in

Lysogene Announces FDA Fast Track Designation for LYS-GM101 Gene Therapy for the Treatment of GM1 Gangliosidosis
Regulatory News:
Lysogene (Paris:LYS) (FR0013233475 – LYS), a gene therapy platform Company targeting central nervous system (CNS) diseases, today announced that the U.S. Food and Drug

GenSight Biologics Announces Approval of the LUMEVOQ® Cohort Temporary Authorization for Use (ATUc) in France
Regulatory News:
GenSight Biologics (Paris:SIGHT) (Euronext: SIGHT, ISIN: FR0013183985, PEA-PME eligible), a biopharma company focused on developing and commercializing innovative gene therapies

GenSight Biologics Reports Topline Results from REFLECT Phase III Clinical Trial, Confirming LUMEVOQ® Efficacy Including Better Efficacy with Bilateral Treatment
Regulatory News:
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20210629006120/en/
Figure 1. Best-Corrected Visual Acuity (BCVA) Change

Cyanotech Reports Financial Results for the Fourth Quarter and Fiscal Year 2021
Cyanotech Corporation (Nasdaq Capital Market: CYAN), a world leader in microalgae-based, high-value nutrition and health dietary supplement products, announced financial results for the fourth

Lysogene Enters into an Exclusive Worldwide License Agreement with SATT Conectus for a Gene Therapy Candidate for the Treatment of the Fragile X Syndrome
Regulatory News:
Lysogene (FR0013233475 – LYS) (Paris:LYS), a phase 3 gene therapy platform Company targeting central nervous system (CNS) diseases, today announces that it has entered into an

GenSight Biologics Appoints Marion Ghibaudo as Chief Technical Officer to Lead GS030 Device Engineering
Regulatory News:
GenSight Biologics (Euronext: SIGHT, ISIN: FR0013183985, PEA-PME eligible), a biopharma company focused on developing and commercializing innovative gene therapies for retinal

Lysogene Announces First Patient Dosed with LYS-GM101 Investigational Gene Therapy for the Treatment of GM1 Gangliosidosis
Regulatory News:
Lysogene (Paris:LYS) (FR0013233475 – LYS), a phase 3 gene therapy platform company targeting central nervous system (CNS) diseases, today announced dosing of the first patient

GenSight Biologics Announces Publication of Indirect Comparison Showing Treatment Effect of LUMEVOQ® versus Natural History in Frontiers in Neurology
Regulatory News:
GenSight Biologics (Paris:SIGHT) (Euronext: SIGHT, ISIN: FR0013183985, PEA-PME eligible), a biopharma company focused on developing and commercializing innovative gene therapies







