News und Analysen

DGAP-Adhoc: BB Biotech AG closes the 2021 fiscal year with a loss

DGAP-News: BB BIOTECH AG: 2021 war ein schwieriges Jahr für die Biotechaktienmärkte - Erhöhung der Dividende auf CHF 3.85 vorgeschlagen

DGAP-News: BB BIOTECH AG: 2021 was a difficult year for biotech equity markets - increased dividend of CHF 3.85 proposed

Kuros Biosciences publishes first-in-human clinical data for Fibrin-PTH (KUR-113) in treatment of open tibial shaft fractures

EQS-Adhoc: Relief Reports US Collaboration Partner Announces Expansion of Aviptadil US Expanded Access & Right to Try Programs for Patients w/COVID-19 Respiratory Failure who've Exhausted All Approved Therapies

EQS-Adhoc: Relief Therapeutics Comments on NRx Pharmaceuticals' Press Release Reporting on its Recently Filed Lawsuit

EQS-Adhoc: Relief Comments on Lawsuit Filed Against It by NeuroRx

EQS-Adhoc: Relief Reports US Collab. Partner announces Submission to FDA seeking EUA for Aviptadil for Pts at Immediate Risk of Death from COVID-19 Despite Treatment w/Remdesivir, Other Approved Therapies

EQS-News: Relief Therapeutics to Participate in Virtual Investor Conferences in January

EQS-Adhoc: Relief Reports that its U.S. Collaboration Partner has Announced the Filing of a Provisional Patent Application for Stable Compositions of Aviptadil Suitable for Human Use

EQS-Adhoc: Relief Therapeutics Announces Notice of Extraordinary General Meeting of RELIEF THERAPEUTICS Holding SA

EQS-Adhoc: Relief Reports U.S. Collab. Partner Announces Filing Breakthrough Therapy Request for Aviptadil in Pts at Immediate Risk of Death from COVID-19 Despite Treatment w/Remdesivir, Other Approved Therapies

EQS-Adhoc: Relief Reports Successful Conclusion of Patent Examination Procedure for Patent Application Entitled, 'Vasoactive Intestinal Peptide (VIP) for the Use in the Treatment of Drug-induced Pneumonitis'

EQS-Adhoc: Evolva secures additional financing lines to accelerate its growth

EQS-Adhoc: Relief Therapeutics Files Registration Statement on Form 20-F with the U.S. Securities and Exchange Commission

EQS-Adhoc: Relief Reports that U.S. Collaboration Partner Announces New, Favorable Safety Report for Aviptadil in NIH Sponsored ACTIV-3b Critical Care Study in Patients with Life-Threatening COVID-19

EQS-Adhoc: Relief Reports that its U.S. Collaboration Partner has Agreed with Hungarian Health Officials on a Pathway for Aviptadil

EQS-Adhoc: Evolva announces private placement of 63.8m shares to VERAISON raising gross proceeds of CHF 7.5m and CFO departure

EQS-Adhoc: Relief Therapeutics Announces Executive Changes

EQS-Adhoc: Relief Reports U.S. Collaboration Partner Identifies Significantly Higher Likelihood of Surviving & Recovering from Critical COVID-19 in Aviptadil Treated Patients Previously Administered Remdesivir

EQS-Adhoc: Relief Therapeutics and InveniAI Sign a Strategic Collaboration Agreement to Identify New Product Development Opportunities using Artificial Intelligence

EQS-Adhoc: Relief Reports that its U.S. Collaboration Partner has Announced the U.S. Food and Drug Administration has Denied Breakthrough Designation for Aviptadil
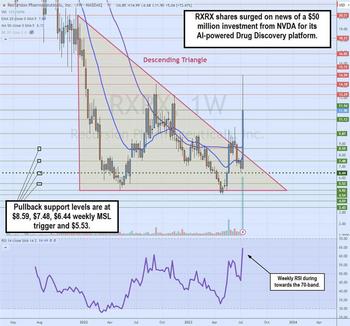
Nvidia Invested $50 million into This AI Drug Discovery Biotech
Clinical-stage biotechnology company Recursion Pharmaceuticals Inc. (NASDAQ: RXRX) saw its stock double on the announcement of a $50 million investment from artificial intelligence (AI) leader

Novartis Stock Screams Value After Chinook Therapeutics Buyout
Don’t be fooled by the fact that Novartis AG (NYSE:NVS) is trading near an all-time high. The Swiss pharmaceutical giant is still a good value.
That value proposition took a huge step forward on
BioNTech präsentiert auf der ASCO-Jahrestagung 2024 klinische Daten-Updates zu innovativen Immuntherapie-Kandidaten
MAINZ, Deutschland, 21. Mai 2024 – BioNTech SE (Nasdaq: BNTX, „BioNTech“ oder „das Unternehmen“) wird auf der diesjährigen Jahrestagung der American Society of Clinical Oncology („ASCO“), die vom






