News und Analysen
BioNTech und OncoC4 starten zulassungsrelevante Phase-3-Studie mit BNT316/ONC-392-Programm bei metastasiertem, nicht-kleinzelligem Lungenkarzinom
- Der Start der zulassungsrelevanten Phase-3-Studie für nicht-kleinzelliges Lungenkarzinom ist ein wichtiger erster Schritt in BioNTechs und OncoC4s strategischer Kollaboration, die im März 2023
BioNTech und OncoC4 präsentieren positive Phase-1/2-Daten für Antikörperkandidaten BNT316/ONC-392 bei schwer behandelbarem nicht-kleinzelligen Lungenkarzinom auf ASCO-Jahrestagung
- BNT316/ONC-392 ist ein innovativer monoklonaler anti-CTLA-4-Antikörperkandidat, der gemeinsam von BioNTech und OncoC4 als Mono- oder Kombinationstherapie zur Behandlung verschiedener solider
3 Stocks Worth Buying at 52-Week Lows
At any time, but in volatile markets particularly, investors who are seeking value can look for stocks that are trading near their 52-week lows. These stocks have a higher likelihood of being

Studie zeigt: Compliance bei indirekten Steuern hat für Marktplatzbetreiber und Verkäufer hohe Priorität, um operative Hürden bei grenzüberschreitenden Verkäufen zu minimieren
LONDON, May 25, 2023 (GLOBE NEWSWIRE) -- Unternehmen nutzen zunehmend Marktplätze für den Online-Verkauf von Produkten und Dienstleistungen. Dabei setzen drei von fünf Unternehmen auf

BioNTech veröffentlicht Ergebnisse des ersten Quartals 2023 und Informationen zur Geschäftsentwicklung
- Fokus im Bereich der COVID-19-Impfstoffe liegt auf Vorbereitungen für eine Impfstoffanpassung vor der Herbstsaison sowie der Entwicklung von Impfstoffkandidaten und -kombinationen der nächsten
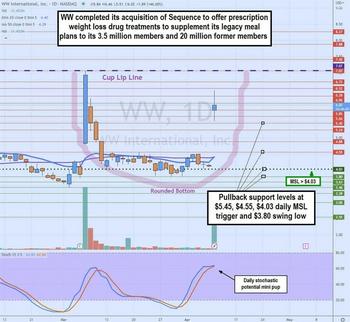
Weight Watchers Reshapes Itself With A Game-Changer Acquisition
Key Points
- Weight Watchers closed its $132 million acquisition of telehealth weight loss platform Sequence Inc. sending shares higher by over 25% on over 37 million shares volume.
- Sequence
Hardman & Co Life Sciences Research: Long-term cost and EBIT analysis of the pharmaceutical industry

Johnson & Johnson Update: ist die Abspaltung gesund oder drohen Probleme?

Abbvie Inc - Technical Analysis

Pfizer Invites Public to View and Listen to Webcast of Pfizer Discussion at Healthcare Conference
Pfizer Inc. (NYSE: PFE) invites investors and the general public to view and listen to a webcast of a discussion with Bob Smith, Vice President, Pfizer Rare Disease, at the 4th Annual Evercore ISI

Follow-Up Data From Phase 3 Trial of Pfizer-BioNTech COVID-19 Vaccine Support Safety and High Efficacy in Adolescents 12 Through 15 Years of Age
Pfizer Inc. (NYSE:PFE) and BioNTech SE (Nasdaq:BNTX) today announced topline results from a longer-term analysis of the safety and efficacy of their COVID-19 vaccine in individuals 12 through 15

Pfizer and BioNTech Receive Expanded U.S. FDA Emergency Use Authorization of COVID-19 Vaccine Booster to Include Individuals 18 and Older
Pfizer Inc. (NYSE: PFE) and BioNTech SE (Nasdaq: BNTX) today announced that the U.S. Food and Drug Administration (FDA) has expanded the emergency use authorization (EUA) of a booster dose of the

Pfizer’s XELJANZ® (tofacitinib) Receives Marketing Authorization in the European Union for the Treatment of Active Ankylosing Spondylitis
Pfizer Inc. (NYSE: PFE) announced today that the European Commission has approved XELJANZ® (tofacitinib) 5 mg twice daily for the treatment of adults with active ankylosing spondylitis (AS) who

Pfizer to Provide U.S. Government with 10 Million Treatment Courses of Investigational Oral Antiviral Candidate to Help Combat COVID-19
Pfizer Inc. (NYSE: PFE) today announced an agreement with the U.S. government to supply 10 million treatment courses of its investigational COVID-19 oral antiviral candidate, PAXLOVID™

Pfizer Completes Acquisition of Trillium Therapeutics
Pfizer Inc. (NYSE: PFE) today announced the successful completion of its acquisition of Trillium Therapeutics, a clinical stage immuno-oncology company developing innovative therapies for the

Pfizer Announces Retirement of Chief Financial Officer Frank D’Amelio
Pfizer Inc. (NYSE: PFE) today announced that Frank D’Amelio will retire from his position as Chief Financial Officer and Executive Vice President of Global Supply at Pfizer after a nearly 15 year

Pfizer Seeks Emergency Use Authorization for Novel COVID-19 Oral Antiviral Candidate
Pfizer Inc. today announced it is seeking Emergency Use Authorization (EUA) of its investigational oral antiviral candidate, PAXLOVID™ (PF-07321332; ritonavir), for the treatment of mild to

Pfizer and The Medicines Patent Pool (MPP) Sign Licensing Agreement for COVID-19 Oral Antiviral Treatment Candidate to Expand Access in Low- and Middle-Income Countries
Pfizer Inc. (NYSE: PFE) and the Medicines Patent Pool (MPP), a United Nations-backed public health organization working to increase access to life-saving medicines for low- and middle-income

Pfizer Aktie: Impfung jetzt überflüssig?

Pfizer Invites Public to Listen to Webcast of Pfizer Discussion at Healthcare Conference
Pfizer Inc. (NYSE: PFE) invites investors and the general public to listen to a webcast of a discussion with Mikael Dolsten, Chief Scientific Officer, President, Worldwide Research, Development and

Pfizer’s Novel COVID-19 Oral Antiviral Treatment Candidate Reduced Risk of Hospitalization or Death by 89% in Interim Analysis of Phase 2/3 EPIC-HR Study
Pfizer Inc. (NYSE: PFE) today announced its investigational novel COVID-19 oral antiviral candidate, PAXLOVID™, significantly reduced hospitalization and death, based on an interim analysis of the

PFIZER REPORTS THIRD-QUARTER 2021 RESULTS
Pfizer Inc. (NYSE: PFE) reported financial results for third-quarter 2021 and raised 2021 guidance(4) for revenues and Adjusted diluted EPS(3) reflecting the net impact of its updated expectations

Pfizer and BioNTech Receive First U.S. FDA Emergency Use Authorization of a COVID-19 Vaccine in Children Ages 5 Through 11 Years
Pfizer Inc. (NYSE: PFE) and BioNTech SE (Nasdaq: BNTX) today announced that the U.S. Food and Drug Administration (FDA) has authorized for emergency use the Pfizer-BioNTech COVID-19 Vaccine for

Pfizer and BioNTech to Provide U.S. Government an Additional 50 Million Pediatric Doses of COVID-19 Vaccine to Support Further Preparedness for Future Needs
Pfizer Inc. (NYSE: PFE) and BioNTech SE (Nasdaq: BNTX) today announced that the U.S. government has purchased 50 million more doses of the companies’ COVID-19 vaccine. The U.S. will receive these

FDA Advisory Committee Votes in Favor of Granting Emergency Use Authorization for the Pfizer-BioNTech COVID-19 Vaccine in Children 5 to <12 Years
Pfizer Inc. (NYSE: PFE) and BioNTech SE (Nasdaq: BNTX) today announced that the U.S. Food and Drug Administration’s (FDA) Vaccines and Related Biological Products Advisory Committee (VRBPAC) voted







.png?locale=de)

