News und Analysen
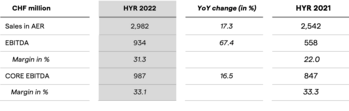
Lonza Delivers Solid Performance in H1 2022 with 16.8% CER Sales Growth and 33.1% CORE EBITDA Margin

DGAP-Adhoc: BB Biotech AG veröffentlicht Zwischenbericht

DGAP-News: BB BIOTECH AG: Erste Anzeichen für vermehrte M&A-Aktivität aufgrund attraktiver Bewertungen

Maria Soler Nunez appointed as Head, Group Operations

DGAP-News: BB BIOTECH AG: Disruptive Technologien verändern einen ganzen Sektor

DGAP-Adhoc: BB Biotech AG veröffentlicht Zwischenbericht

DGAP-News: BB BIOTECH AG: Etablierte Kernbeteiligungen ermöglichen einen erhöhten Investitionsgrad

Vifor Pharma supports Iron Deficiency Day 2021: Call to action for early iron deficiency diagnosis
Already for the seventh consecutive year of Iron Deficiency Day, Vifor Pharma is supporting a growing international alliance including the Heart Failure Policy Network, European Kidney Health

Vifor Pharma to spearhead development of vascular calcification field, through acquisition of Sanifit Therapeutics and Inositec AG
Regulatory News:
AD HOC ANNOUNCEMENT PURSUANT TO ART. 53 LR
Vifor Pharma has announced the acquisition of Sanifit Therapeutics, a Spanish clinical-stage cardio-renal biopharmaceutical company

VFMCRP receives positive CHMP opinion for Tavneos® for the treatment of ANCA-associated vasculitis
Regulatory News:
AD HOC ANNOUNCEMENT PURSUANT TO ART. 53 LR
Vifor Fresenius Medical Care Renal Pharma (VFMCRP) today announced that the European Medicines Agency’s (EMA) CHMP has recommended

Vifor Pharma and Angion report topline results from phase-III registration trial of ANG-3777 in kidney transplant patients at risk for delayed graft function
Regulatory News:
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20211026006150/en/
AD HOC ANNOUNCEMENT PURSUANT TO ART. 53 LR
Vifor

Vifor Pharma announces changes to its Executive Committee
Regulatory News:
AD HOC ANNOUNCEMENT PURSUANT TO ART. 53 LR
Vifor Pharma Group today announced changes to its Executive Committee as current members Lee Heeson, President International, and

VFMCRP announces approval for TAVNEOS® (avacopan) for the treatment of ANCA-associated vasculitis in Japan
Vifor Fresenius Medical Care Renal Pharma (VFMCRP) today announced that Japan’s Ministry of Health and Labor Welfare (MHLW) has granted its partner, Kissei Pharmaceutical Co., Ltd., marketing

GeNeuro and Northwestern University Enter Into a Research Collaboration on HERV-W ENV in Long-haul COVID
GeNeuro (Paris:GNRO) (Euronext Paris: CH0308403085 - GNRO), a biopharmaceutical company developing new treatments for neurodegenerative and autoimmune diseases, such as multiple sclerosis

Vifor Pharma and Travere Therapeutics announce licensing agreement for the commercialization of sparsentan in Europe, Australia and New Zealand
Regulatory News:
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20210915005966/en/
AD HOC ANNOUNCEMENT PURSUANT TO ART. 53 LR
Vifor

Vifor Pharma’s Ferinject® granted new recommendations in updated 2021 ESC heart failure guidelines
Regulatory News:
Vifor Pharma is pleased to announce that the European Society of Cardiology (ESC) included new recommendations and proposals in the 2021 ESC guidelines for the diagnosis and

Vifor Pharma and Cara Therapeutics announce U.S. FDA approval of KORSUVA™ injection for the treatment of moderate-to-severe pruritus in hemodialysis patients
Regulatory News:
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20210823005635/en/
Vifor Pharma and Cara Therapeutics (Nasdaq:CARA)

Vifor Pharma reports strong H1 2021 growth, on track to meet full year guidance1
Regulatory News:
Vifor Pharma Group reported profitable revenue growth in H1 supported by a strong recovery in Ferinject® / Injectafer® with sales up 22.8% at CER as a result of improved patient

Vifor Pharma erzielt im 1. Halbjahr 2021 starkes Wachstum und bestätigt Jahresprognose1
Regulatory News:
Die Vifor Pharma Gruppe erzielte im 1. Halbjahr 2021 ein profitables Umsatzwachstum dank einer starken Erholung von Ferinject®/Injectafer® mit einem Umsatzplus von 22.8% zu kWk

Abbas Hussain Appointed as New Chief Executive Officer of Vifor Pharma
Regulatory News:
Abbas Hussain (56, British citizen) has a successful and international career of more than 30 years as an executive in the healthcare sector. From 2008 to 2017, Abbas Hussain

Abbas Hussain zum neuen Chief Executive Officer von Vifor Pharma ernannt
Regulatory News:
Abbas Hussain (56, britischer Staatsbürger) verfügt über eine mehr als 30-jährige erfolgreiche und internationale Karriere als Führungskraft im Gesundheitssektor. Von 2008 bis

Vifor Pharma to revise DIAMOND study, readout expected in H2 2021
Regulatory News:
Vifor Pharma Group today announced that the phase-IIIb DIAMOND study has been amended with new and clinically relevant endpoints, including a new primary endpoint of efficacy in

93rd Vifor Pharma Group Annual General Meeting
Regulatory News:
At today’s 93rd Annual General Meeting of Vifor Pharma Ltd., shareholders approved all proposed resolutions put forward by the Board of Directors. In view of the ongoing COVID-19

93. Generalversammlung der Vifor Pharma Gruppe
Regulatory News:
An der 93. ordentlichen Generalversammlung der Vifor Pharma AG haben die Aktionäre alle Beschlussanträge des Verwaltungsrats genehmigt. Angesichts der anhaltenden

VFMCRP announces positive results of phase-III clinical trial of Velphoro® in China
Regulatory News:
Vifor Fresenius Medical Care Renal Pharma (VFMCRP) today announced positive results from a phase-III study in China (PA-CL-CHINA-01), evaluating the efficacy of Velphoro® (PA21)







