News

Valbiotis announces the launch of a capital increase

Valbiotis announces the availability of an amendment to the Universal Registration Document

Valbiotis announces that it has received Food and Drug Administration (FDA) approval for New Dietary Ingredient (NDI) status

VALBIOTIS SA:

Valbiotis announces the success of the TOTUM•63 mode of action clinical study, against prediabetes and the early stages of type 2 diabetes

VALBIOTIS SA: the Phase II/III REVERSE-IT study selected for an oral presentation at the 2023 EASD congress, the main European learned society for diabetes

Valbiotis publishes its financial report for the first half of 2023 and confirms its strategic roadmap

VALBIOTIS SA: Valbiotis presents the full results of the Phase II/III REVERSE-IT study: impressive efficacy of TOTUM•63 against prediabetes and the early stages of type 2 diabetes

Valbiotis announces its roadmap and strategic priorities on the eve of key milestones for its portfolio of innovative active substances

Valbiotis announces the appointment of Charlotte JEZEQUEL as Director of Human Relations and Executive Committee member
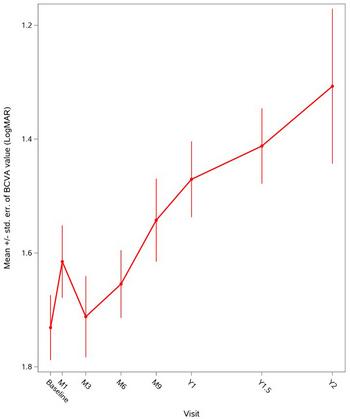
GenSight Biologics Announces Presentation of LUMEVOQ® Efficacy and Safety Data from Early Access Programs for ND4-LHON Patients at NANOS 2023
Regulatory News:
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20230314006021/en/
Figure 1: Global evolution of mean BCVA over two
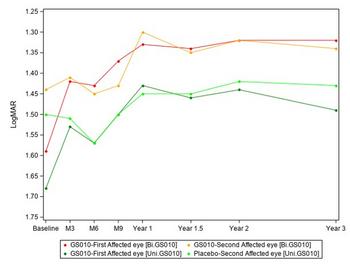
GenSight Biologics Confirms Sustained Efficacy and Safety of Bilateral LUMEVOQ® Injections at 3-Year Follow-Up of REFLECT Phase III Trial
Regulatory News:
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20230312005028/en/
Graph 1: Evolution of Best-Corrected Visual Acuity
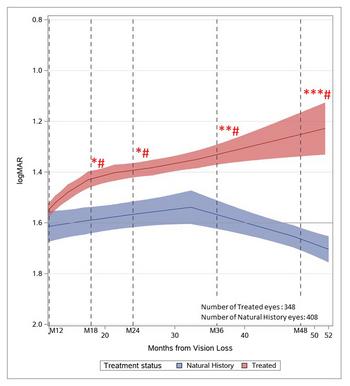
GenSight Biologics Announces Publication of Indirect Comparison of LUMEVOQ® Versus Natural History in ND4-LHON Patients in Peer-Reviewed Journal Ophthalmology and Therapy
Regulatory News:
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20221214006010/en/
Figure 1: from Indirect Comparison of Lenadogene
Form 8.3 - The Vanguard Group, Inc.: Sanofi
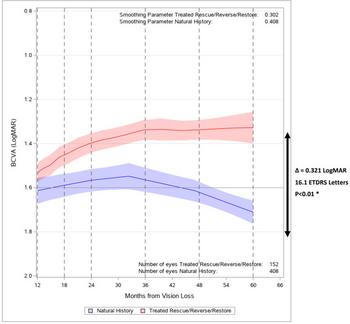
GenSight Biologics reports 5 years’ data showing sustained efficacy and safety following one-time treatment with LUMEVOQ®
Regulatory News:
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20220719006077/en/
Figure 1. Evolution of BCVA In LUMEVOQ®-treated

Transgene’s Board of Directors Strengthens Its Governance and Proposes the Appointment of Dr. Alessandro Riva as Independent Chairman
Regulatory News:
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20220331005765/en/
Dr. Alessandro Riva (Photo: Transgene)
Transgene

GenSight Biologics Reports Clinically Meaningful Vision Improvement is Maintained 4 Years After One-time Treatment with LUMEVOQ® Gene Therapy
Regulatory News:
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20220123005091/en/
Figure 1. Evolution of BCVA In LUMEVOQ®-treated

GenSight Biologics Confirms Sustained Efficacy and Safety of Bilateral LUMEVOQ® Injections at 2-Year Follow-Up of REFLECT Phase III Trial
Regulatory News:
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20211213005948/en/
Figure 1. Best-Corrected Visual Acuity (BCVA) Change

Sartorius Stedim Biotech SA: DECLARATION RELATIVE TO THE NUMBER OF SHARES AND VOTING RIGHTS MAKING UP THE ISSUED CAPITAL

Poxel Provides a Financial Update for the First Quarter 2024 and Announces the Postponement of its 2023 Full-Year Results Release
Regulatory News:
POXEL SA (Euronext: POXEL - FR0012432516) (Paris:POXEL), a clinical stage biopharmaceutical company developing innovative treatments for chronic serious diseases with metabolic

Sensorion Announces its Participation in the American Society of Cell and Gene Therapy (ASGCT) Annual Meeting
Regulatory News:
Sensorion (FR0012596468 – ALSEN) a pioneering clinical-stage biotechnology company which specializes in the development of novel therapies to restore, treat and prevent within

GenSight Biologics Announces the Filing of its 2023 Universal Registration Document
Regulatory News:
GenSight Biologics (Euronext: SIGHT, ISIN: FR0013183985, PEA-PME eligible), a biopharma company focused on developing and commercializing innovative gene therapies for retinal

Sartorius Stedim Biotech publishes unaudited first quarter results for 2024

Sensorion Announces its Participation in the Van Lanschot Kempen's Life Sciences Conference
Regulatory News:
Sensorion (FR0012596468 – ALSEN) a pioneering clinical-stage biotechnology company which specializes in the development of novel therapies to restore, treat and prevent within

Sensorion Announces a €15 Million Financing, Extending Cash Runway Until the End of 2025
Regulatory News:
Sensorion (FR0012596468 – ALSEN) a pioneering clinical-stage biotechnology company which specializes in the development of novel therapies to restore, treat and prevent hearing





