News

DGAP-News: Abivax Includes First Patient in U.S. Phase 1/2 Clinical Trial of ABX196 to Treat Hepatocellular Carcinoma

Sartorius Stedim Biotech SA: Information on Document Availability

Sartorius Stedim Biotech SA: DECLARATION RELATIVE TO THE NUMBER OF SHARES AND VOTING RIGHTS MAKING UP THE ISSUED CAPITAL

DGAP-News: Abivax to Host Satellite Symposium at 15th Congress of the European Crohn's and Colitis Organization (ECCO) in Vienna

DGAP-News: Abivax veranstaltet Satellite-Symposium auf dem 15. Kongress der 'European Crohn's and Colitis Organisation' (ECCO) in Wien

DGAP-News: Abivax erhält FDA-Genehmigung für den Start klinischer Studien mit ABX464 in den USA zur Behandlung mittelschwerer bis schwerer Colitis ulcerosa

DGAP-News: Abivax Receives Clearance from U.S. FDA to Initiate Clinical Trials with ABX464 to treat Moderate to Severe Ulcerative Colitis

DGAP-News: ABIVAX: Finanzkalender 2020

Pharnext Announces Encouraging Data from Open-Label Phase 3 Extension Study of PXT3003 in Charcot-Marie-Tooth Disease Type 1A (CMT1A)

Correction of a release from 02.01.2020, 09:55 CET/CEST - Sartorius Stedim Biotech SA: ANNUAL REPORT OF THE LIQUIDITY CONTRACT WITH THE STOCKBROKER COMPANY GILBERT DUPONT

Sartorius Stedim Biotech SA: ANNUAL REPORT OF THE LIQUIDITY CONTRACT WITH THE STOCKBROKER COMPANY GILBERT DUPONT

Sartorius Stedim Biotech SA: DECLARATION RELATIVE TO THE NUMBER OF SHARES AND VOTING RIGHTS MAKING UP THE ISSUED CAPITAL

Sartorius Stedim Biotech SA: DECLARATION RELATIVE TO THE NUMBER OF SHARES AND VOTING RIGHTS MAKING UP THE ISSUED CAPITAL

Pharnext establishes an equity line facility with Kepler Cheuvreux

Sartorius Stedim Biotech SA: DECLARATION RELATIVE TO THE NUMBER OF SHARES AND VOTING RIGHTS MAKING UP THE ISSUED CAPITAL

Pharnext announces 2019 half-year results

DGAP-News: Biom'up announces the approval of HEMOBLAST(TM) Bellows and its laparoscopic applicator in Australia

Valbiotis publishes its annual accounts 2023

Valbiotis to launch its 100% natural dietary supplement for the management of hypercholesterolemia on the French market in May
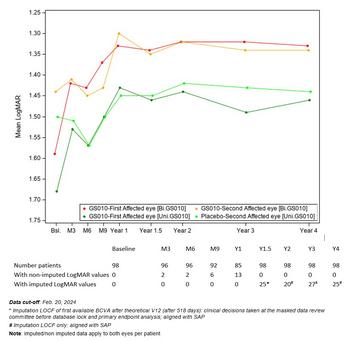
GenSight Biologics Confirms Sustained Efficacy and Safety of Bilateral LUMEVOQ® Injections Four Years After One-Time Administration
Regulatory News:
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20240319984906/en/
Graph 1: Evolution of Best-Corrected Visual Acuity
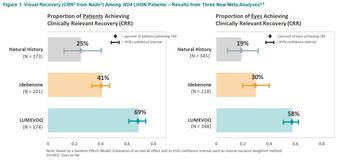
GenSight Biologics Announces Initial Results from New Meta-Analyses on Visual Outcomes with LUMEVOQ® Gene Therapy at NANOS 2024
Regulatory News:
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20240311531952/en/
Figure 1. Visual Recovery (CRR from Nadir) Among
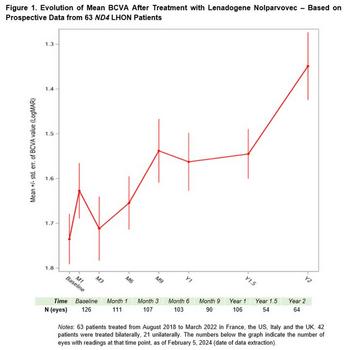
GenSight Biologics Announces Update on Real-World Data from Early Access Programs of LUMEVOQ® Gene Therapy at NANOS 2024
Regulatory News:
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20240305810568/en/
(Graphic: Business Wire)
GenSight Biologics

Valbiotis presents its 2024 financial communication calendar

Valbiotis sets out its commercial and clinical roadmap for 2024







