News

Why MannKind, Nutanix, and Smith & Nephew Jumped Today

Form 8.3 - The Vanguard Group, Inc.: NMC Health plc

Form 8.3 - The Vanguard Group, Inc.: NMC Health plc

Form 8.3 - The Vanguard Group, Inc.: NMC Health plc

Form 8.3 - The Vanguard Group, Inc.: NMC Health plc

Form 8.3 - The Vanguard Group, Inc.: NMC Health plc

Form 8.3 - The Vanguard Group, Inc.: NMC Health plc

Form 8.3 - The Vanguard Group, Inc.: NMC Health plc

Form 8.3 - The Vanguard Group, Inc.: NMC Health plc

Form 8.3 - The Vanguard Group, Inc.: NMC Health plc

Form 8.3 - The Vanguard Group, Inc.: NMC Health plc

Form 8.3 - The Vanguard Group, Inc.: NMC Health plc

Form 8.3 - The Vanguard Group, Inc.: Consort Medical plc

Form 8.3 - The Vanguard Group, Inc.: Consort Medical plc

Form 8.3 - Link Fund Solutions Ltd:HVIVO PLC

Form 8.3 - The Vanguard Group, Inc.: Consort Medical plc

Form 8.3 - The Vanguard Group, Inc.: Consort Medical plc

Form 8.3 - The Vanguard Group, Inc.: Consort Medical plc

Form 8.3 - The Vanguard Group, Inc.: Consort Medical plc

Form 8.3 - The Vanguard Group, Inc.: Consort Medical plc

Form 8.3 - Consort Medical Plc
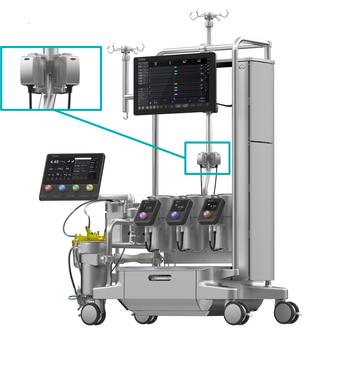
LivaNova präsentiert Essenz In-Line Blood Monitor mit U.S. FDA 510(k)-Zulassung und CE-Prüfzeichen
LivaNova PLC (Nasdaq: LIVN), ein Marktführer im Bereich der Medizintechnik, gab heute bekannt, dass es die 510(k)-Zulassung der US-amerikanischen Food and Drug Administration (FDA) und das
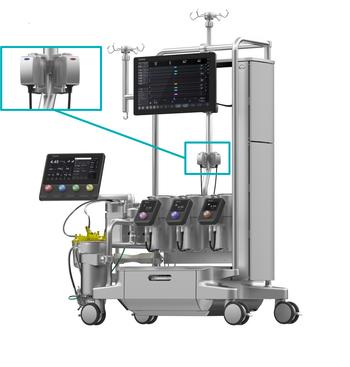
LivaNova Unveils Essenz In-Line Blood Monitor with U.S. FDA 510(k) Clearance and CE Mark
LivaNova PLC (Nasdaq: LIVN), a market-leading medical technology company, today announced it received U.S. Food and Drug Administration (FDA) 510(k) clearance and CE Mark for its Essenz™ In-Line

LivaNova erhält 510(k)-Freigabe der US-amerikanischen FDA für Essenz-Herz-Lungen-Maschine für kardiopulmonale Bypassverfahren
LivaNova PLC (Nasdaq: LIVN), ein marktführendes Unternehmen im Bereich medizintechnische Innovationen, gab heute bekannt, dass es die 510(k)-Freigabe von der US-amerikanischen Food and Drug

LivaNova Receives U.S. FDA 510(k) Clearance for Essenz Heart-Lung Machine for Cardiopulmonary Bypass Procedures
LivaNova PLC (Nasdaq: LIVN), a market-leading medical technology and innovation company, today announced it received U.S. Food and Drug Administration (FDA) 510(k) clearance for its Essenz™







